Annual Integrated Report | Takeda Pharmaceuticals
Annual Integrated Report
2023 Annual Integrated Report
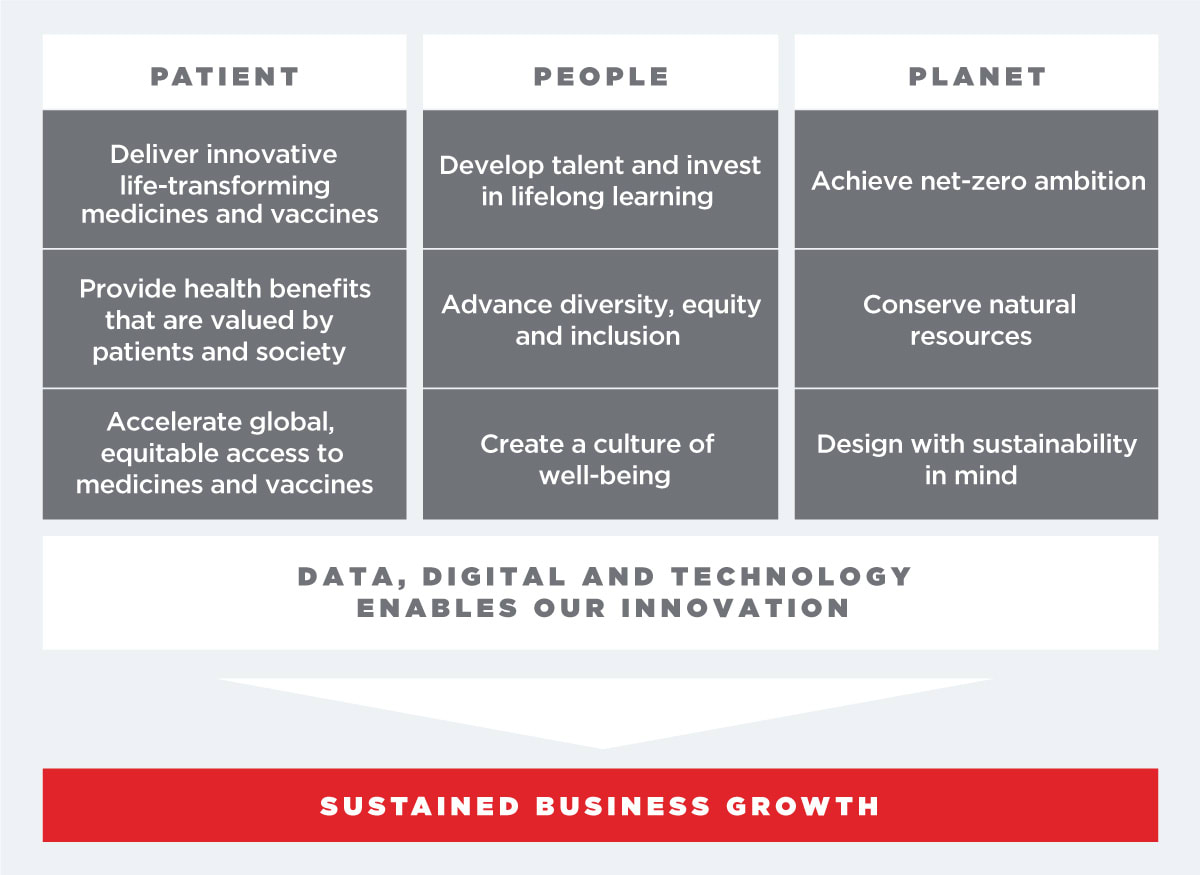
Sustainability Framework
We developed our corporate philosophy imperatives: Patient, People and Planet, which identify where Takeda must invest to deliver on our purpose, informed by an ESG materiality assessment of strategically important nonfinancial issues to our company and stakeholders. In FY2022, we evolved these imperatives to create a simplified sustainability framework and sustainability focus areas.
We create long-term value through our progress toward these imperatives and sustainability focus areas, which we measure through our corporate philosophy metrics. More details on these metrics, including why they matter, can be found in the Annual Integrated Report.
KPMG AZSA Sustainability Co., Ltd., undertook limited assurance on our corporate philosophy metrics. Assured content can be found below.
Highlights from FY2022
Here are some FY2022 achievements taken from our 2023 Annual Integrated Report. Learn more about our efforts and progress in the report.